Deep Biology Platform
Why Tregs?
Regulatory T cells, or Tregs, are a special subset of T cells that act as key regulators of the immune system. In the tissues, Tregs are important players in tissue inflammation and the mediation of tissue healing.
By studying Tregs we can better understand how those regulatory pathways affect other tissue immune and stromal cells.
Tregs modulation is key to therapeutic innovation and TRexBio is at the forefront, building a robust pipeline of transformative medicines addressing autoimmune and inflammatory diseases.
What Makes our Platform Different?
At the core of our approach is the realization that correcting disease processes at the tissue level leads to better clinical outcomes. We know that immune system imbalance plays a significant role in the development of disease, but in-depth studies of immune processes in human tissue have historically been limited.
The Traditional Approach
-

Murine biology
-

Validation in peripheral blood
-

Therapeutic molecule
Previous drug discovery efforts have relied on peripheral blood and/or mouse models which have had only limited translatable insights.
The TRex Approach
-

Human tissue biology
-
Computational biology and functional fingerprinting
-

Therapeutics built on human tissue-specific hypotheses
At TRexBio we start with human tissue, where disease actually occurs.
With the advancement of functional genomics and sequencing technologies, and the increasing ability to process and interpret large quantities of high-dimensional data, we now have the tools to interrogate human tissue processes at unprecedented resolution.
TRexBio is at the forefront of these efforts, combining multidisciplinary expertise across immunobiology, functional genomics, and computational and translational biology to uncover the ‘deep biology’ in human tissues.
Three Pillars of the TRexBio Deep Biology Platform
The foundation of our platform is a high-resolution map of tissue immune regulation in health and disease derived from our broad collection of human tissue. Our powerful discovery platform maps tissue Treg behavior to disease processes and identifies novel biology for therapeutic intervention.
Computational Mapping
Computational Mapping
We use advanced in silico tools to identify key pathways in tissue regulation, leveraging a proprietary human tissue bank and atlas of human Treg gene expression to pinpoint drivers of disease and therapeutic opportunity. This approach allows us to uncover the most relevant biology for therapeutic intervention, faster and with greater precision than traditional methods.
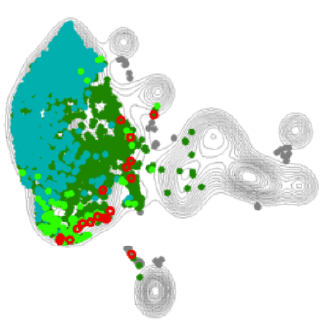
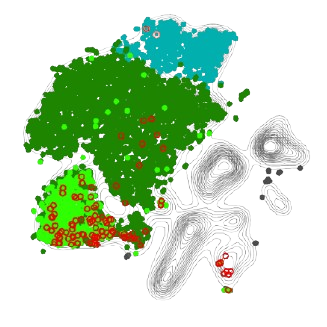
An example of our Treg-enriched scRNA sequencing output across two different skin diseases, highlighting a tissue-Treg enriched pathway in red.
TReg Atlas
TReg Atlas
Our proprietary Treg Atlas recreates tissue states in Treg, capturing approximately 1,000 genes enriched in tissue-resident Tregs. We use this data to reprogram blood-derived Tregs and recreate human tissue-specific pathways in vitro, offering a reliable model for downstream target validation.
There are over
1000
tissue Treg enriched genes
Of all human T cells
95%
reside and function in tissues
Phenotypic Assay Cascade
Phenotypic Assay Cascade
Our Assay Cascade is a suite of high-throughput functional fingerprints optimized for human biology. By testing targets across multiple donors and inflammatory contexts, we generate translational data that inform target validation, candidate selection, and clinical positioning.



Deep Biology Platform
We use our novel platform to produce translatable insights for the initiation, validation, and advancement of novel drug discovery programs.
Access to targets unreachable by traditional methods
Rapid functional validation of therapeutic hypotheses
Deep understanding of target and disease biology
A pipeline of selective, tissue-targeted Treg modulators
