TRB-061
TRB-061: A TNFR2 Agonist for Atopic Dermatitis and Other Inflammatory Diseases
TRB-061 is designed to reduce inflammation and promote barrier tissue repair by selectively expanding a highly activated subpopulation of Tregs that are preferentially located in the tissue. TRB-061 is in clinical development for moderate-to-severe atopic dermatitis (AD).
A Novel Mechanism in Immune Regulation
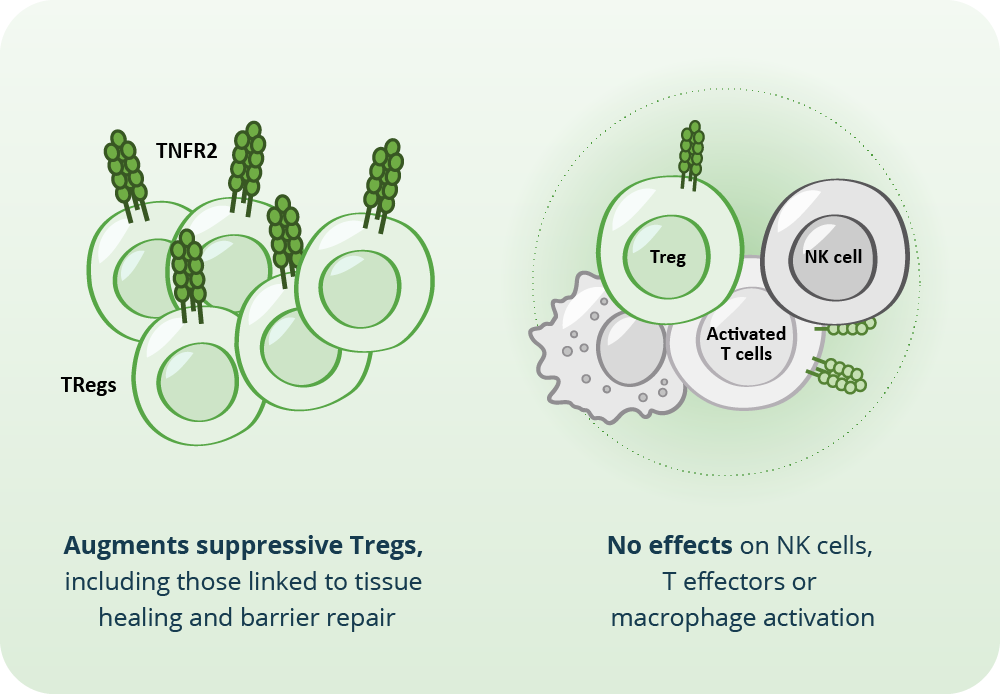
TNFR2 is a key activator of effector Tregs in inflamed tissues. These cells are critical to controlling immune responses and maintaining tissue health. TRB-061 mimics the natural TNF ligand to selectively activate effector Tregs.
Phase 1 Clinical Trial in Progress
The phase 1a/1b randomized, double-blind, placebo-controlled, 3-part study will investigate the safety, pharmacokinetics, and pharmacodynamics of single and multiple subcutaneous doses of TRB-061 in healthy participants and in patients with moderate-to-severe atopic dermatitis (Clinicaltrials.gov Identifier: NCT06934252)
Unlike traditional anti-inflammatories or systemic IL-2-based therapies, which can have broad immune activation liabilities, TRB-061 is designed to act locally and selectively, without activating macrophages, NK cells, or effector T cells.
In atopic dermatitis, these suppressive Tregs are present but underactive, and express high TNFR2, making them potentially responsive to TRB-061’s mechanism. By selectively agonizing TNFR2 on Treg, TRB-061 is designed to expand and reactivate these tissue-resident Tregs, which we believe may be an improved approach to long-term disease control.
TRB-061 is designed to reduce inflammation and promote barrier tissue repair by selectively expanding a highly activated subpopulation of Tregs that are preferentially located in the tissue. TRB-061 is in clinical development for moderate-to-severe atopic dermatitis (AD).
A Novel Mechanism in Immune Regulation
TNFR2 is a key activator of effector Tregs in inflamed tissues. These cells are critical to controlling immune responses and maintaining tissue health. TRB-061 mimics the natural TNF ligand to selectively activate effector Tregs.
Unlike traditional anti-inflammatories or systemic IL-2-based therapies, which can have broad immune activation liabilities, TRB-061 is designed to act locally and selectively, without activating macrophages, NK cells, or effector T cells.
In atopic dermatitis, these suppressive Tregs are present but underactive, and express high TNFR2, making them potentially responsive to TRB-061’s mechanism. By selectively agonizing TNFR2 on Treg, TRB-061 is designed to expand and reactivate these tissue-resident Tregs, which we believe may be an improved approach to long-term disease control.
Phase 1 Clinical Trial in Progress
TRB-061 for Precision Immunomodulation
TRB-061 is a clinical-stage, TNFR2-selective agonist designed to restore immune balance within inflamed tissues through targeted Treg augmentation, offering a differentiated approach from anti-inflammatory or IL-2-based strategies with the potential to improve care in atopic dermatitis and other autoimmune diseases.
Atopic Dermatitis: Transformative Immunomodulation
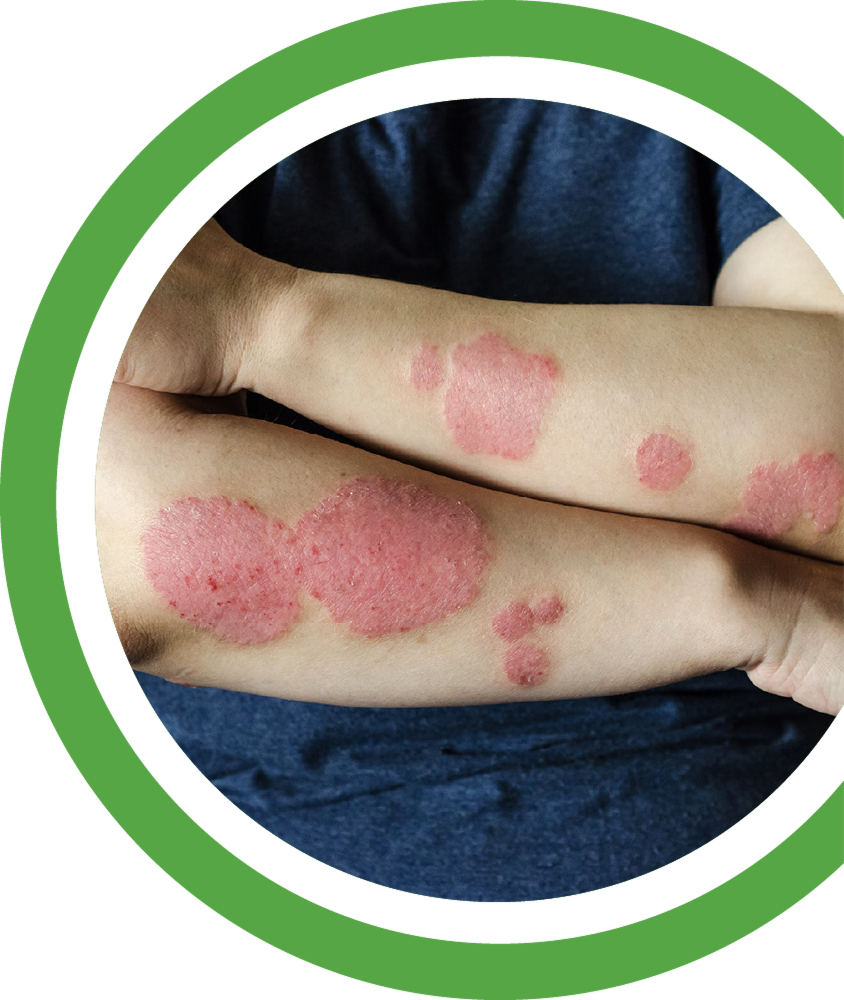
Atopic dermatitis (AD) is a chronic inflammatory skin disease characterized by rash, itching, and skin barrier dysfunction. AD affects up to 20% of children and 10% of adults, affecting approximately 204 million worldwide. In moderate-to-severe cases, AD is associated with systemic immune activation, extensive body surface area involvement, and substantial quality-of-life impairment.
While approved biologics have improved outcomes for some patients, more than half discontinue current therapies within two years due to ineffectiveness and long-term side effects. TRB-061 aims to fill the therapeutic gap between the safety of current biologics and the deeper efficacy of more aggressive agents, without systemic immune suppression.